Classification of Matter
In Science, matter is anything that has mass and takes up space. all the "stuff" around you is matter, and you are matter too. Chemistry is the study of matter and how matter changes.
Some types of matter are substances and some are not. In chemistry, a substance is a single kind of matter that is pure, meaning it always has a specific makeup or composition.
Every form of matter has two kinds of properties, physical and chemical properties. A physical properties is a characteristic of a substance that can be observed without changing it into another substance. A chemical property is a characteristic of a substance that describes its a ability to change into different substances.
Atom
What the smallest thing you can think of? A grain of sand? A speck of dust? If you look at these items under a powerful microscope, you'll see that they're made up of smaller and smaller pieces. All matter is made up of very tiny particles called atoms. Atoms are the basic building blocks of everything you can see around you, and even a lots of things that you can not see around you, like the air that you breath. Atoms are very small that there are billions in the tiniest speck you can see. Solids, liquids, and gases (all matters) are made up af atoms, molecules also are made up of atoms.Some types of matter are substances and some are not. In chemistry, a substance is a single kind of matter that is pure, meaning it always has a specific makeup or composition.
Every form of matter has two kinds of properties, physical and chemical properties. A physical properties is a characteristic of a substance that can be observed without changing it into another substance. A chemical property is a characteristic of a substance that describes its a ability to change into different substances.
Atom
At the center of the atom is a tiny, dense nucleus containing protons (+) and neutrons. surrounding the nucleus is a cloudlike region of moving electrons (-).
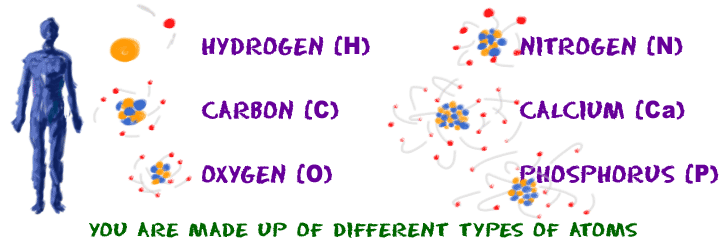
Elements
If you want to build molecules you will need elements with different of atoms. Elements are the kinds of atoms that we can have. Elements also is a pure substance that cannot be separated into simpler substance by physical or chemicals activity. You have billions of atoms in your body. However, you may only find about 91 elements.
Each element has a unique chemical name and symbol. The chemical symbol consists of one, two, or three letters. The firs letter is always capitalized and the remaining letter(s) are always lower case. All elements are listed out in the periodic table. For example Aluminium has symbol Al in the periodic table. To see periodic table click this link http://pslc.ws/macrog/kidsmac/periodic.htm
There are three groups of elements based on its properties, including melting temperature, density, hardness, thermal and electrical conductivity: metal, metalloid, and non metal. Metal are elements that are good conductors of electric current and heat. They also tend to be shiny and bendable-like cooper wire, for instance. Metal are the most element in periodic table. A non-metal is an element that lacks most of the properties of a metal. In general, most non-metals are poor conductors of electric current and heat. Solid non-metals tend to be dull and brittle
There are three groups of elements based on its properties, including melting temperature, density, hardness, thermal and electrical conductivity: metal, metalloid, and non metal. Metal are elements that are good conductors of electric current and heat. They also tend to be shiny and bendable-like cooper wire, for instance. Metal are the most element in periodic table. A non-metal is an element that lacks most of the properties of a metal. In general, most non-metals are poor conductors of electric current and heat. Solid non-metals tend to be dull and brittle
Picture 2: Elements
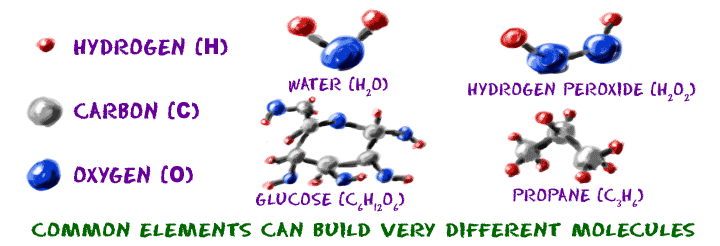
Molecules
Molecules are groups of atoms bonded together is the same way that words are groups of letters. An "A" will always an "A" no matter what words it is in. A sodium (Na) atom will always a sodium atom no matters what compound it is in. For example, to make molecule of Hydrogen (H2), you need two atoms of hydrogen (H) or two elements of hydrogen (H) (in element of hydrogen only have one atom). 

Molecules can be much bigger. One molecule of vitamin C is made up of 20 atoms (6 carbons, 8 hydrogens, and 6 oxygens - that's C6H8O6). If you take those 20 atoms of vitamin C and mix them around, bonding them together in a different order, you will have a totally different molecule that not only looks different, it acts different.
Vitamin C Another molecule
C6H8O6 C6H8O6
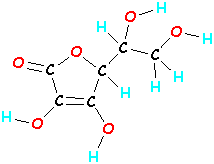

Picture 3: Molecules of Vitamin
Compound
A Compound is combination of two or more different elements that are combined chemically. Water, salt, sugar, and aspirin are example of common compounds. Today, there are approximately 10 million known compounds, and new compounds continue to be developed and discovered at the rate of about 100.000 per year. The chemical symbols of periodic table make it easy to write the formulas for chemical compounds. For example salt or sodium chloride, is composed of one part sodium (Na) and one part chlorine (Cl), and its chemical formula is NaCl. Water is composed of two parts hydrogen (h) and one part oxygen (O), and its formula is H2O.
When elements chemically combine, they form compounds with properties different from those of the elements. The element sulfur is a yellow solid and the element copper is a shiny metal, when copper and sulfur combine, they form a compound called copper sulfide. The new compound has different properties from both copper and sulfur.
When elements chemically combine, they form compounds with properties different from those of the elements. The element sulfur is a yellow solid and the element copper is a shiny metal, when copper and sulfur combine, they form a compound called copper sulfide. The new compound has different properties from both copper and sulfur.
Mixtures
A Mixture is made two or more substances that are together in the same place, but their atom are not chemically bonded. Mixture differ from compounds, each substance in a mixture keeps its own properties.
Based on the distribution of their components, mixture can be classified as Homogeneous Mixture (Solution), is a mixture in which the composition is uniform, the substance involved in a homogeneous mixture are so every mixed that you can't see the different parts. It is difficult to separate the parts of a homogeneous mixture.
Example: Sugar dissolved in water. Sugar serves as the dissolved substance (solute), while water serves as the solvent. In a solution, the solute has a relatively small particle size, so there will be no sediment when it is being left to sit for considerable time.
Heterogeneous Mixture (Suspension), is a mixture in which the composition is not uniform, you can usually see the different parts and they can easily be separated out.
Example: Muddy water, at a glance muddy water looks like a solution, however if it is left for a time, the mud will settle at the bottom of the container while clear water will be left in the upper part of the container.
Colloids, is a mixture which has dissolved particles smaller than in a suspension but bigger than in a solution. Colloids appear to be homogeneous, however we can still differentiate every dissolved particle inside the colloid using an ultra microscope.
Example: Milk, coconut milk, jelly paint, and soap water.
A Mixture is made two or more substances that are together in the same place, but their atom are not chemically bonded. Mixture differ from compounds, each substance in a mixture keeps its own properties.
Based on the distribution of their components, mixture can be classified as Homogeneous Mixture (Solution), is a mixture in which the composition is uniform, the substance involved in a homogeneous mixture are so every mixed that you can't see the different parts. It is difficult to separate the parts of a homogeneous mixture.
Example: Sugar dissolved in water. Sugar serves as the dissolved substance (solute), while water serves as the solvent. In a solution, the solute has a relatively small particle size, so there will be no sediment when it is being left to sit for considerable time.
Heterogeneous Mixture (Suspension), is a mixture in which the composition is not uniform, you can usually see the different parts and they can easily be separated out.
Example: Muddy water, at a glance muddy water looks like a solution, however if it is left for a time, the mud will settle at the bottom of the container while clear water will be left in the upper part of the container.
Colloids, is a mixture which has dissolved particles smaller than in a suspension but bigger than in a solution. Colloids appear to be homogeneous, however we can still differentiate every dissolved particle inside the colloid using an ultra microscope.
Example: Milk, coconut milk, jelly paint, and soap water.
From simple to complex
If you think deeper, imagine the smallest particles of matter. Super tiny subatomic particles are used to create the parts of atoms. Protons, neutrons, and electrons can then organize to form atoms. Atoms are then used to create the molecules around us, there are a lot of elements that can be found in the molecules we know. Smaller molecules can work together and build macromolecules. It just goes on. You can start really small.
- Particles of matter
- Atoms
- Molecules
- Macromolecules (compound)
- Cell organelles
- Cells
- Tissue
- Organs
- Organ system
- Organism
- Populations
- Ecosystems
- Biomes
- Planets
- Planetary system
- Galaxies
- Universe
That all become possible because of atoms.
Based on the characteristic of the particles, mixture can be classified into solutions, suspensions, and colloids. Scientist have developed different methods of separating mixture, so that we can get the useful substances we need from them.
1. Filtration
Filtration is conducted using filter with holes or pores. The size of the pores or holes on the filter depends on the size of the substance and the purpose of the separation of the mixture.
a. Water purification
Is a one method of filtration.
1. Filtration
Filtration is conducted using filter with holes or pores. The size of the pores or holes on the filter depends on the size of the substance and the purpose of the separation of the mixture.
a. Water purification
Is a one method of filtration.
Picture 5: Water Filtration
b. Centrifugation
Centrifugation is done by spinning the mixture of suspension at high speed.
2. Evaporation
Sometimes a solution cannot be separated by filtration because its soluble particles are relatively small. To separate such a solution we can use evaporation. In this method, we heat the solute substance and its solvent until the solvent boils and evaporates.
The separation of two substances by distillation is done based on the boiling point differences between the two substances. If a mixture of substances with different boiling points is heated, the substance with the lower boiling point will be the first to boil and evaporate. Afterwards, we collect and cool it until it condenses. The drops from the condensation process are the pure substance called distillate. Meanwhile, the substance with the higher boiling point has not evaporated yet. We can use mixture separation by distillation to separate both solid-liquid mixtures and liquid-liquid mixtures.
Centrifugation is done by spinning the mixture of suspension at high speed.
Picture 6: Centrifugation
2. Evaporation
Sometimes a solution cannot be separated by filtration because its soluble particles are relatively small. To separate such a solution we can use evaporation. In this method, we heat the solute substance and its solvent until the solvent boils and evaporates.
Picture 7: Evaporation
3. DistillationThe separation of two substances by distillation is done based on the boiling point differences between the two substances. If a mixture of substances with different boiling points is heated, the substance with the lower boiling point will be the first to boil and evaporate. Afterwards, we collect and cool it until it condenses. The drops from the condensation process are the pure substance called distillate. Meanwhile, the substance with the higher boiling point has not evaporated yet. We can use mixture separation by distillation to separate both solid-liquid mixtures and liquid-liquid mixtures.
Picture 8: Distillation
4. Sublimation
You have learned that two types of liquid with different boiling points can be separated through distillation. One of the way to separate two solid substance is through sublimation. Sublimation is conducted when there are two solid substance, and one substance can sublime when it is heated. Example: Camphor, iodine and ammonium chloride.
Picture 9: Sublimation of Iodine
5. Separation using a separator funnel
You can separate a mixture composed of two types of solution which do not dissolve one another using separatory funnel. Example, you can separate the mixture between water and oil.
Picture 10: Separatory funnel
6. Chromatography
Different colours are mixed together to make inks and paints. these colours can be separated using a method called chromatography.
Picture 11: Chromatography
References:
Dingrando, Laurel. Chemistry: Matter and Change. Glencoe/Macgraw-Hill. 2002: United State
"Atoms, Elements, and Molecules." Atoms, Elements, and Molecules. N.p., n.d. Web. 08 Oct. 2014.
Morrison, Karen. International Science. Hodder Education. 2008: UK, London
Sumarwan. Science for Junior High School 1st Semester Grade VII. Erlangga. 2006: Jakarta
Buckley, Don. Introduction to Chemistry: Interactive Science. Pearson. 2011: United State
Dingrando, Laurel. Chemistry: Matter and Change. Glencoe/Macgraw-Hill. 2002: United State
"Atoms, Elements, and Molecules." Atoms, Elements, and Molecules. N.p., n.d. Web. 08 Oct. 2014.
Morrison, Karen. International Science. Hodder Education. 2008: UK, London
Sumarwan. Science for Junior High School 1st Semester Grade VII. Erlangga. 2006: Jakarta
Buckley, Don. Introduction to Chemistry: Interactive Science. Pearson. 2011: United State